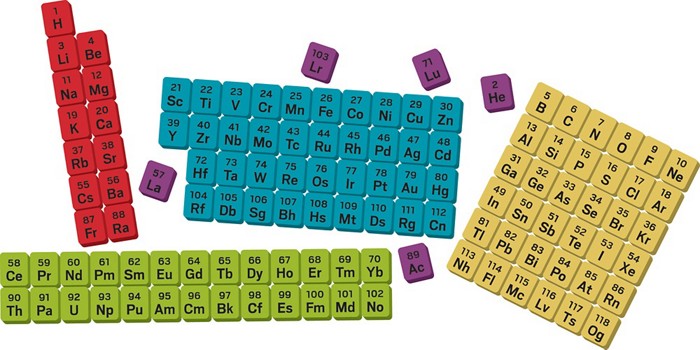
Before proceeding with the Naming Elements with Atomic Number 100 and More, let us first understand the modern periodic table. After that, we shall try to understand the role of the International Union of Pure and Applied Chemistry (IUPAC) and how the atomic name is assigned. The Modern Periodic Table consists of 118 elements discovered so far.
To give honour to the discoverer of the new elements, the new chemical name is given the name of the eminent scientists to give due credit to their research work in the field of science. The IUPAC ratifies it by approving the scientific name, and then the naming of the elements is further considered.
Modern Periodic Table and Properties of Elements –
The modern periodic table gives us insights into the various physical and chemical properties of these elements. As per the laws, they are the periodic functions of their respective atomic numbers. Thus, scientists and researchers from allied fields arranged these elements in the increasing order of the atomic numbers from the left to the right side.
This is done across each row. They even discovered that some of the elements with similar properties repeat after some regular intervals.
IUPAC and Naming of Elements –
- Out of the 118 elements, 24 are developed synthetically, while the remaining are found naturally. Some of the elements discovered include the one by Gen Seaborg and his team in California.
- Some of them are assigned with nomenclatures or names along with symbols. However, some of these names and symbols are not universally used.
- To quote an example, the element with the atomic number 104 is attributed to both American and Russian scientists. The Russians named it ‘Ku’ for Kurchatovium; the Americans named it ‘Rf’ for Rutherfordium.
- Similarly, the atomic number 107 is being assigned the nomenclature of ‘Ns’ for Neilsbohrium and ‘Bh’ for Bohrium.
- However, these discrepancies in assigning the nomenclature are done away with by the IUPAC (International Union of Pure and Applied Chemistry).
- To overcome the difficulties of naming elements, IUPAC came up with recommendations for elements with atomic numbers greater than 100.
- IUPAC mandated that the names of these elements should use Latin words and symbols to describe them. Also, the names would arrive from the Latin numerical roots.
In 1978, IUPAC formed a commission to align clear rules of elements nomenclature on systematic lines. The commission formed was called the CNIC (Commission on Nomenclature of Inorganic Chemistry).
CNIC established clear rules for assigning names or nomenclature to newly discovered elements with atomic numbers greater than 100. Elements having atomic numbers greater than 100 are also known as super-heavy elements because of their characteristics.
Nomenclature of Atomic Elements –
The general principle is that when a discoverer finds a new element and decides to give it a name, he must follow some IUPAC guidelines.
After that, the name is approved based on public opinion. It is then given a temporary name as provided under the IUPAC guidelines. The naming continues till IUPAC recognises and gives it a permanent formal name to the element.
Also, the CNIC established that the names of the elements should be named systematically by following the below points-
- The nomenclature should be short and should be in relation to the element’s atomic number.
- The nomenclature should end with ‘ium’ whether the element belongs to the non-metal or the metal groups.
- The elements symbol in systematic naming nomenclature consists of 3 alphabets.
- The element’s symbol consists of the initial alphabets of the respective numerical roots.
Moreover, let us try to understand the set of guidelines given by IUPAC for naming atomic elements.
1) The nomenclature of the element is derived from the atomic number of the element with the use of the below Latin numerical roots –
| Number | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Root | nil | un | bi | tri | quad | pent | hex | sept | oct | enn |
| Abbreviation | n | u | b | t | q | p | h | s | o | e |
2) The above roots are arranged together in the order of their digits that tells us the element’s atomic number. Its name ends with ‘ium’. Two general principles are followed here –
- If the name occurs before ‘nil’, the last letter ‘n’ in the root ‘enn’ is omitted.
- If a ‘bi’ or ‘tri’ occurs before the root ‘ium’, then the ‘i’ is removed.
3) The most important aspect is that the element’s symbol is composed of the initial letters of the given numerical roots to arrive at its name.
4) The numerical root ‘un’ is pronounced in a way that rhymes with ‘uno’.
Some of the nomenclatures of elements with atomic numbers greater than or equal to 100 are listed below: –
| Atomic number | Name | Temporary Name | Name of the Element | Symbol |
| 101 | Unnilunium | Unu | Mendelevium | Md |
| 102 | Unnilbium | Unb | Nobelium | No |
| 103 | Unniltrium | Unt | Lawrencium | Lr |
| 104 | Unnilquadium | Unq | Rutherfodium | Rf |
| 105 | Unnilpentium | Unp | Dubnium | Db |
| 106 | Unnilhexium | Unh | Seaborgium | Sg |
| 107 | Unnilseptium | Uns | Bohrium | Bh |
| 108 | Unniloctium | Uno | Hassium | Hs |
| 109 | Unnilennium | Une | Meitnerium | Mt |
| 110 | Ununnilium | Uun | Darmstadtium | Ds |
| 111 | Unununium | Uuu | Roentgenium | Rg |
| 112 | Ununbium | Uub | Copernicium | Cn |
| 113 | Ununtrium | Uut | Nihonium | Nh |
| 114 | Ununquadium | Uuq | Flevorium | Fl |
| 115 | Ununpentium | Uup | Moscovium | Mc |
| 116 | Ununhexium | Uuh | Livermorium | Lv |
| 117 | Ununseptium | Uus | Tennessine | Ts |
| 118 | Ununoctium | Uuo | Oganesson | Og |
More Interesting facts
The element Fermium with atomic number 100 and symbol ‘Fm’ is a synthetic element. It is one of the heaviest elements that can be prepared by the bombardment of neutrons of some lighter elements. It is thus manufactured in minuscule quantities, although it does not exist in nature.
Again, we all have heard about Uranium which is a very unstable element that occurs in nature. It can also be created in the physics laboratory. However, scientists have been able to create elements until the atomic number 118 as they are highly unstable. It is also one of the heaviest elements that occur in nature and different forms.
Conclusion
The periodic table is the backbone of Chemistry and plays a vital role in finding out the characteristics of each element. To memorise them, students need to identify different parts of each element type using their similarities in properties.
You can also memorise or learn a few elements a day then gradually master them over time. It is recommended that students print out the periodic table and stick them on the walls adjacent to their study tables. This will ensure that they memorise and learn daily.